Навигация
2.1 Gaseous Diffusion
The gaseous diffusion process has been used to enrich nearly all of the low and highly enriched uranium that has been produced in the United States. It was first developed in the 1940s as part of the Manhattan Project and was used to enrich a portion of the uranium used in the bomb that was dropped on Hiroshima. All five acknowledged nuclear weapons states within the nuclear non-proliferation treaty (NPT) regime have operated gaseous diffusion plants at one time or another, but currently only the United States and France continue to operate such facilities. The diffusion process requires pumping uranium in a gaseous form through a large number of porous barriers and, as noted above, is very energy intensive.
In order to make the uranium into a gaseous form that can be used in the diffusion process, the natural uranium is first converted into uranium hexafluoride (UF6). The uranium hexafluoride molecules containing U-235 atoms, being slightly lighter, will diffuse through each barrier with a slightly higher rate than those containing U-238 atoms. A simple analogy to help visualize this process is to imagine blowing sand through a series of sieves. The smaller grains of sand will preferentially pass through each sieve, and thus after each stage they would represent a slightly higher percentage of the total than they did before passing through the stage. A schematic representation of one such stage from a gaseous diffusion plant is shown in Figure 1.

Figure 1: Schematic diagram of a single stage in a gaseous diffusion plant.
The darker colors represent the UF6 molecules that contain the heavier U-238 atoms, while the lighter colors represent gas molecules that contain the lighter U-235. After each stage the gas to the low pressure side of the barrier (i.e. the downstream side) has a slightly higher percentage of U-235 than the stage before.
The difference in mass, and therefore velocity, between the UF6 molecules containing either U-235 or U-238 is very small, and thus thousands of such stages are needed in order to enrich commercial or military amounts of uranium. In a gaseous diffusion plant, the stages are arranged into “cascades” that allow each stage to build on the enrichment achieved by the ones before it and also to more efficiently make use of the depleted uranium stream. For a sense of scale, when it was first constructed in the early 1940s the gaseous diffusion plant at Oak Ridge, Tennessee, was the largest industrial building in the world. The facility at Oak Ridge is shown in Figure 2 while a picture of two of the diffusers used in the enrichment process is shown in Figure 3.
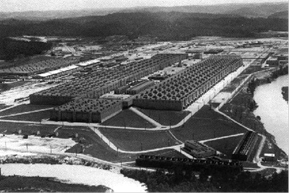
Figure 2: Oak Ridge gaseous diffusion plant, built during World War II.
At the time of its construction this was the largest industrial building in the world. In part it was decided to locate this plant in Tennessee so that its large electricity demand could be met by the abundant coal and hydroelectric plants built by the government run Tennessee Valley Authority. It is now closed and awaiting decommissioning.
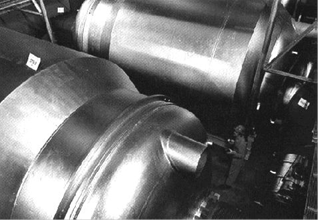
Figure 3: A close up picture of the outside of two of the diffuser stages used at the Oak Ridge uranium enrichment plant.
The diffusers contain the porous barriers used to separate the lighter U-235 atoms from the heavier U-238 atoms. Connected to the diffusers is equipment to compress the uranium hexafluoride gas and pipe it through the cascade as well as equipment to remove the large amount of heat generated during the enrichment process. Each diffuser and compressor are together referred to as a “stage.”
The most challenging step in building a gas diffusion plant is to manufacture the permeable barriers required in the diffusers. The material for the barriers needs to be highly durable and able to maintain a consistent pore diameter for several years of operation. This is particularly challenging given the highly corrosive nature of the uranium hexafluoride gas used. Typical barriers are just 5 millimeters (less than 0.2 inches) thick and have openings that are only about 30 to 300 times the diameter of a single uranium atom.
In addition to requiring a large amount of electricity during operation, the compressors in the gas diffusion facilities also generate a great deal of heat that requires dissipation. In U.S. plants this heat is dissipated through the use of ozone depleting chlorofluorocarbons (CFCs) such as the coolant CFC-114 (often referred to simply as Freon of Freon-114). The manufacture, import, and use of CFCs were substantially restricted by the 1987 Montreal Protocol on Substances That Deplete the Ozone Layer, which the U.S. is implementing through the 1990 Amendments to the Clean Air Act. As a result of these commitments, the manufacture of Freon in the U.S. ended in 1995 and its emissions to the air in the United States from large users fell by nearly 60% between 1991 and 2002.The emissions from the Paducah gaseous diffusion plant, however, have remained virtually constant over this time, falling just over 7% between 1989 and 2002.In 2002, the Paducah enrichment plant emitted more than 197.3 metric tons of Freon into the air through leaking pipes and other equipment. This single facility accounted for more than 55% of all airborne releases of this ozone depleting CFC from all large users in the entire United States in 2002.Due to the lack of additional manufacturing of Freon since 1995, the U.S. Enrichment Corporation is currently looking for a non-CFC coolant to use. Likely candidates would still have heat trapping potential, and thus even if they were not as dangerous to the ozone layer, they would still remain a potential concern in relation to global warming and climate change.
The high heat signature of gaseous diffusion plants makes it possible that plants operating significantly in excess of 100 MTSWU per year could be detected. However, this information would likely only be meaningful as a way of identifying operations at known plants and not for uncovering clandestine facilities since there are many industrial processes that generate a great deal of heat. Thus, while gaseous diffusion plants are perhaps one of the hardest types of uranium enrichment facility to hide given their size, electricity needs, and heat signature, it would still be difficult to remotely identify a facility without access to environmental samples from the surrounding area.
Похожие работы
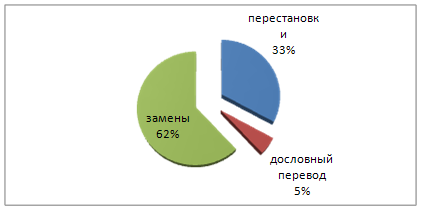
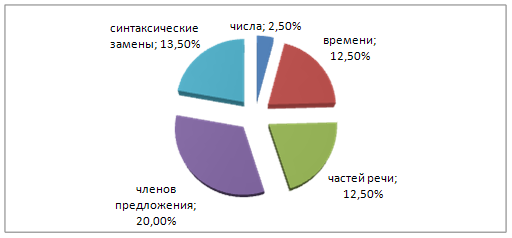
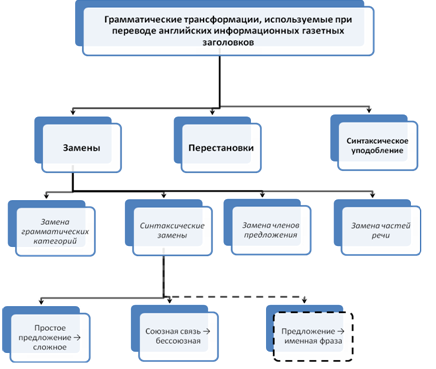
... - замены + замены грамматических категорий + замены части речи + замены членов предложения + синтаксические замены + синтаксическое уподобление (дословный перевод) + Грамматические трансформации, используемые при переводе английских информационных газетных заголовков Замены, используемые при переводе английских информационных газетных заголовков ...
of promoting a morpheme is its repetition. Both root and affixational morphemes can be emphasized through repetition. Especially vividly it is observed in the repetition of affixational morphemes which normally carry the main weight of the structural and not of the denotational significance. When repeated, they come into the focus of attention and stress either their logical meaning (e.g. that of ...
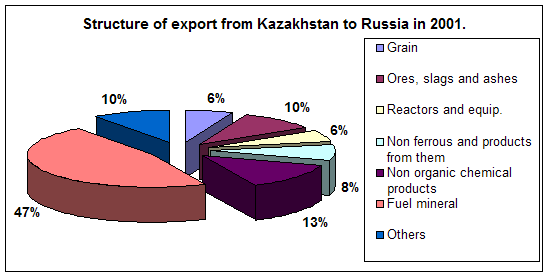
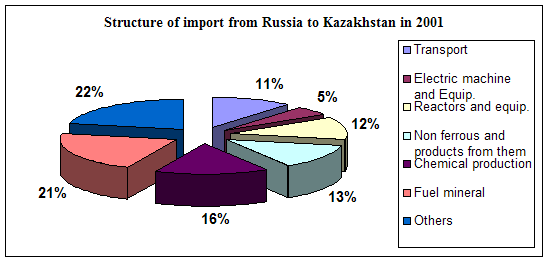
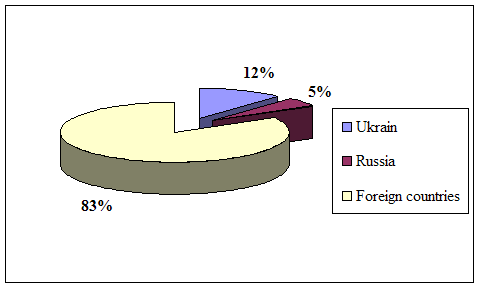
... course of republic. A complex of the reasons conditions and factors having not tactical, but basic essence and long-time character stipulates it. Today common balance of mutual relation between Kazakhstan and Russia has positive character, as consider each other as the strategic partners and it establishes the important premise for their mutual cooperating in the field of policy, economy, ...
... long coils of thin copper wire...., a) he was disappointed b) on opposite sides of the ring 10. Составить предложение: by, produce, wanted, electricity, Faraday, to, electromagnetism. 11. Перевести на английский язык: 1) Идея получения тока непосредственно действием магнита не оставляла его мыслей, 2) Обычно Фарадей носил в кармане маленькую модель электромагнитного прибора. 12. Ответить на ...
0 комментариев