Навигация
Review
Aluminium
Content
1. Introduction
2. Characteristics
3. Isotopes
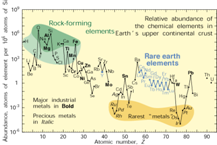
4. Natural occurrence
5. Production and refinement
6. Recycling
7. Chemistry
7.1 Oxidation state +1
7.2 Oxidation state +2
7.3 Oxidation state +3
7.4 Analysis
8. Applications
8.1 General use
8.2 Aluminium compounds
8.3 Aluminium alloys in structural applications
8.4 Household wiring
9. History
10. Etymology
10.1 Nomenclature history
10.2 Present-day spelling
11. Health concerns
12. Effect on plants
13. Conclusion
14. References
1. Introduction
Aluminium is a silvery white and ductile member of the boron group of chemical elements. It has the symbol Al; its atomic number is 13. It is not soluble in water under normal circumstances. Aluminium is the most abundant metal in the Earth's crust, and the third most abundant element therein, after oxygen and silicon. It makes up about 8% by weight of the Earth’s solid surface. Aluminium is too reactive chemically to occur in nature as a free metal. Instead, it is found combined in over 270 different minerals.[4] The chief source of aluminium is bauxite ore.
Aluminium is remarkable for its ability to resist corrosion due to the phenomenon of passivation and for the metal's low density. Structural components made from aluminium and its alloys are vital to the aerospace industry and very important in other areas of transportation and building. Its reactive nature makes it useful as a catalyst or additive in chemical mixtures, including being used in ammonium nitrate explosives to enhance blast power.
General properties
Name, symbol, number aluminium, Al, 13
Element category other metal
Group, period, block 13, 3, p
Standard atomic weight 26.9815386(13) g·mol−1
Electron configuration [Ne] 3s2 3p1
Electrons per shell 2, 8, 3 (Image)
Physical properties
Phase solid
Density (near r.t.) 2.70 g·cm−3
Liquid density at m.p. 2.375 g·cm−3
Melting point 933.47 K, 660.32 °C, 1220.58 °F
Boiling point 2792 K, 2519 °C, 4566 °F
Heat of fusion 10.71 kJ·mol−1
Heat of vaporization 294.0 kJ·mol−1
Specific heat capacity (25 °C) 24.200 J·mol−1·K−1
Vapor pressure
P/Pa 1 10 100 1 k 10 k 100 k
at T/K 1482 1632 1817 2054 2364 2790
Atomic properties
Oxidation states 3, 2[1], 1[2]
(amphoteric oxide)
Electronegativity 1.61 (Pauling scale)
Ionization energies
(more) 1st: 577.5 kJ·mol−1
2nd: 1816.7 kJ·mol−1
3rd: 2744.8 kJ·mol−1
Atomic radius 143 pm
Covalent radius 121±4 pm
Van der Waals radius 184 pm
Miscellanea
Crystal structure face-centered cubic
Magnetic ordering paramagnetic[3]
Electrical resistivity (20 °C) 28.2 nΩ·m
Thermal conductivity (300 K) 237 W·m−1·K−1
Thermal expansion (25 °C) 23.1 µm·m−1·K−1
Speed of sound (thin rod) (r.t.) (rolled) 5,000 m·s−1
Young's modulus 70 GPa
Shear modulus 26 GPa
Bulk modulus 76 GPa
Poisson ratio 0.35
Mohs hardness 2.75
Vickers hardness 167 MPa
Brinell hardness 245 MPa
CAS registry number 7429-90-5
2. Characteristics
Aluminium is a soft, durable, lightweight, malleable metal with appearance ranging from silvery to dull grey, depending on the surface roughness. Aluminium is nonmagnetic and nonsparking. It is also insoluble in alcohol, though it can be soluble in water in certain forms. The yield strength of pure aluminium is 7–11 MPa, while aluminium alloys have yield strengths ranging from 200 MPa to 600 MPa.[5] Aluminium has about one-third the density and stiffness of steel. It is ductile, and easily machined, cast, drawn and extruded.
Corrosion resistance can be excellent due to a thin surface layer of aluminium oxide that forms when the metal is exposed to air, effectively preventing further oxidation. The strongest aluminium alloys are less corrosion resistant due to galvanic reactions with alloyed copper.[5] This corrosion resistance is also often greatly reduced when many aqueous salts are present however, particularly in the presence of dissimilar metals.
Aluminium atoms are arranged in a face-centred cubic (fcc) structure. Aluminium has a stacking-fault energy of approximately 200 mJ/m².[6]
Aluminium is one of the few metals that retain full silvery reflectance in finely powdered form, making it an important component of silver paints. Aluminium mirror finish has the highest reflectance of any metal in the 200–400 nm (UV) and the 3000–10000 nm (far IR) regions, while in the 400–700 nm visible range it is slightly outdone by tin and silver and in the 700–3000 (near IR) by silver, gold, and copper.[7]
Aluminium is a good thermal and electrical conductor, by weight better than copper. Aluminium is capable of being a superconductor, with a superconducting critical temperature of 1.2 kelvin and a critical magnetic field of about 100 gauss.[8]
Похожие работы
... . The advertising idea is defined. Advertising strategy is however insufficiently clearly stated. We will take advantage of the given reserve for increase of efficiency of an advertising campaign of Open Company "Натали", i. e. we will develop advertising strategy. 3.3 Use of methods of optimization in advertising activity One of optimisation methods in advertising activity is use of ...
... in 1975 together with Paul Alien, his partner in computer language development. While attending Harvard in 1975, Gates together with Alien developed a version of the BASIC computer programming language for the first personal computer. In the early 1980s. Gates led Microsoft's evolution from the developer of computer programming languages to a large computer software company. This transition ...
... , weight, our distinctive marks.That are shown in the blank enclosed. Plz observe all these requirements or we’ll have to refuse the offer with no charges payed. Ex 2: Write a letter for your firm to an English engineering firm, ordering a special machine. Give packing and marking instructions. thx u for offer of april 1.we r very interested in this sort of machine & would like to order 5 ...
... of lithium were produced through the electrolysis of lithium chloride by Robert Bunsen and Augustus Matthiessen.[20] The discovery of this procedure henceforth led to commercial production of lithium metal, beginning in 1923 by the German company Metallgesellschaft AG, which performed an electrolysis of a liquid mixture of lithium chloride and potassium chloride.[20][29] The production and use ...




0 комментариев