Measuring Specific Latent Heat of Vaporization of Water
Introduction:
The aim of this experiment was to determine the specific latent heat of vaporization of a liquid using basic equipment and calculations. The accepted value of the latent vaporization heat is 2.3x 104 Jkg-1. Hopefully the results obtained will be similar to the accepted value.
Apparatus:
Kettle
3 beam Scales
Stop watch
Variables:
Method:
1. Take the kettle and fill it up half way with water
2. Put it on the scales and measure the mass
3. Record the mass in the data table
4. Turn on the kettle
5. When the water starts to boil start timing
6. Stop timing when 50 grams of water have been lost
7. Record in the data table
8. Repeat steps 1-7 five times
Data Collection and Analysis
Time of the trials
| Trials | Mass/kg ± 0.005 | Time/s ± 5s |
| 1 | 0.050 | 187 |
| 2 | 0.050 | 180.0 |
| 3 | 0.050 | 180.0 |
| 4 | 0.050 | 192 |
| 5 | 0.050 | 145 |
| Average | 0.050 | 186 |
Uncertainties
The scientist determined the uncertainties by measuring them. The scientist wasn’t sure when to start timing the boiling water, and as there was no set time time written in the procedure , so the uncertainty was determined to be 5s. As the scales used to measure the mass of the water were not electronic, but three beam, the minimum uncertainty was determined to be 5 grams . The uncertainty for the specific latent heat of vaporization was based on the uncertainties of mass and time.
Pt=mLv 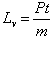
Trial 1 m=0.05 t=187s
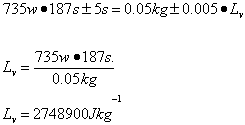
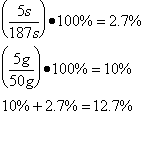
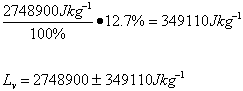
Trial 2 m=0.05 t=180s
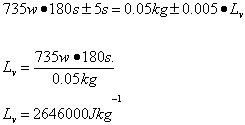
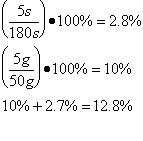
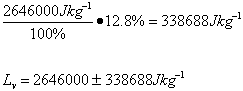
Trial 3 m=0.05g t=180s
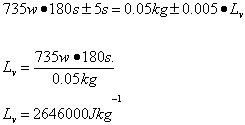
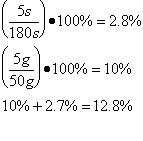
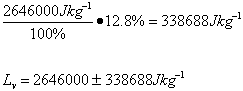
Trial4 m=0.05kg t=192s
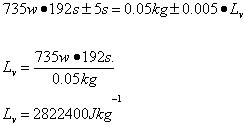
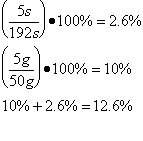
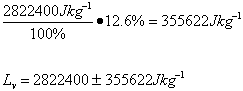
Trial 5 m=0.05kg t=189s
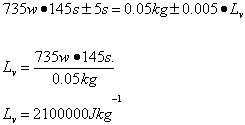
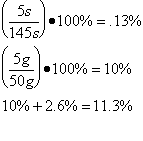
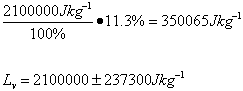
Average
| Trials | 1 | 2 | 3 | 4 | 5 |
| Lv /Jkg-1 | 2.7 x 106 ±349110 | 2.6 x 106 ±338688 | 2.8 x 106 ±355622 | 2.6 x 106 ±338688 | 2.1 x 106 ±237300 |
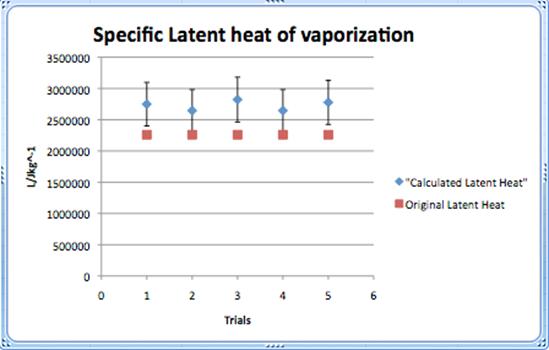
Conclusions and evaluations
The calculated value of the specific latent heat of vaporization was supposed to be 2.3 x 106 J/Kg. Unfortunately the results achieved by the calculations were not perfect and did not reach the accepted values. All the Lv calculated were in one range, but did not come close to 2.3 x 106.
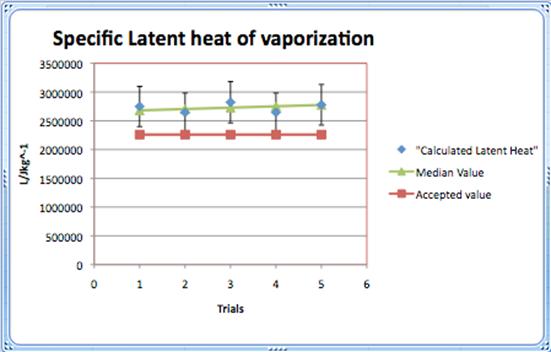
The median value of the calculated Latent heat of vaporization was modelled by the equation
y= 25520x+2657760
and showed that the values were still inflated, but regarding the uncertainty, some values reached the accepted value.
The reasons of such weak results were the uncertainties of the procedure, and old equipment. The scientist didn’t have a set time to start timing the boiling water, therefore the time could be the reason of the misleading calculations. The scales that the scientist was usingwere three-beam, and not electronic. This could as well be a reason of the uncertain mass. It was difficult for the scientst to measure the exact moment,when the water had evaporated 50 grams of water.
Reasonable improvements for this experiment would be, setting a time for the scientist to start measuring
0 комментариев